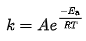Arrhenius equation (아레니우스 식) 속도 상수의 온도 의존성을 수식으로 나타낸 식 - k = rate constant (frequency of collisions resulting in a reaction) - T = absolute temperature (in Kelvin) - A = pre-exponential factor (arrhenius originally considered A to be a temperature-independent constant for each chemical reations. However, more recent treatments include some tmperature dependence) - Ea = activation energy for the ..